The Highest Standards from Tissue Selection through Processing
From start to finish, quality is at the forefront of everything we do. MTF Biologics maintains the most stringent donor screening process of any tissue bank – exceeding the industry standards all tissue banks must follow. But our commitment to quality doesn’t end there.
MTF Biologics believes in minimal processing to maintain the biomechanical integrity and biochemistry of every graft. Our unique aseptic processing method ensures optimal tissue quality, by minimizing the use of harsh chemicals and terminal radiation.

Tissue You Can Trust
In conjunction with our distribution partners, Orthofix, Depuy Synthes, Kolosis, and ConMed, MTF Biologics offers one of the largest portfolios of orthopedic and sports medicine tissues, ranging from bone void fillers like Trinity ELITE® a viable cell allograft to our clinically tested and proven tendons for ACLR’s.
Our notable orthopedic tissue solutions include:
- Broad Range of Bone Void Fillers - Trinity Elite®, Trinity Evolution, fiberFUSE, Kore Fiber®, DBX and Chips
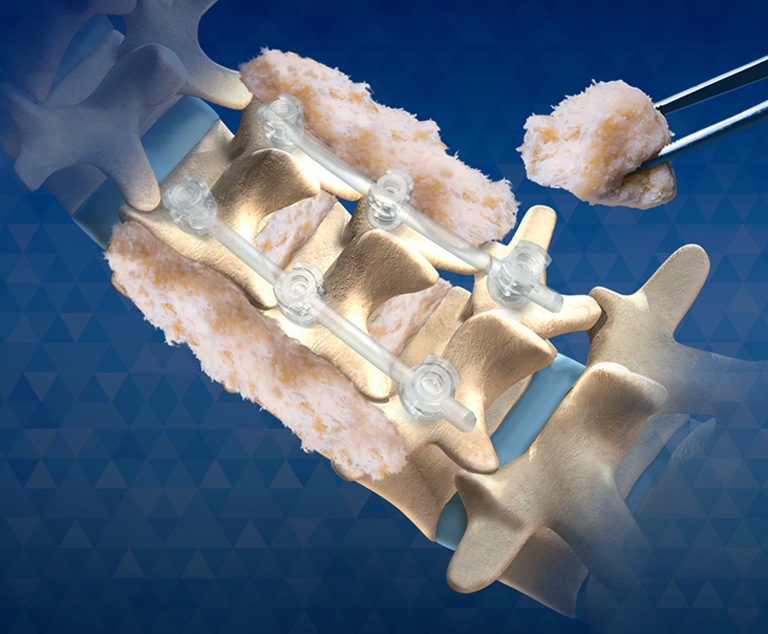
- Structural Allograft Spinal Spacers - Largest portfolio for ACDF's, ALIF's, TLIF's, PLIF's and Lateral Procedural grafts
- Allograft Tendons - Large portfolio of soft tissue, bone-tendon-bone and pre-shaped grafts for the treatment of sports medicine injuries
- Amniotic Tissues - Anti-adhesion barrier
Trinity ELITE®
Allograft with Viable Cells with FiberLock™ Technology
A True Autograft Substitute:
Contains the three essential components for bone formation
- Osteoconductive:
Osteoconductive scaffold is provided by the cancellous bone component. It provides a porous interconnected scaffold for bone ingrowth. The trabecular matrix (cancellous bone)is the optimal micro environment for bone cells to attach, proliferate, and remodel. - Osteoinductive:
Growth factors inherent in the demineralized cortical fibers are necessary in the bone healing cascade. The growth factors are known to induce bone formation by recruiting osteoprogenitor cells, differentiating cells down the bone forming pathway and promoting angiogenesis. - Osteogenic:
Osteogenic cells include viable adult MSCs, OPCs and bone forming cells such as osteoblasts that are native to the cancellous matrix. Cells which attach to the DBM surface are able to interface with the growth factors resulting in a positive effect on bone formation.
FiberLock Technology:
- Acts as a scaffold to provide bone-to-bone contact for graft containment
- Withstand blood flow once implanted and resists washing away
- Interlocking fibers deliver versatile moldability and exceptional handling with no carrier added

Matching the Right Graft to the Right Patient
Size appropriate and procedure specific grafts are crucial to what you do. And it’s important to us, too. That’s why MTF Biologics offers matching for our large grafts, meniscal, and fresh osteochondral allografts, with the goal of providing the best anatomically matched allograft tissue for your patients. This exceptional service saves you valuable surgical time, by reducing extensive modifications during transplantation. Once we achieve a match, we discuss all information and data with you for final acceptance.
- Prompt, same-day service for most requests
- One-on-one consultation with a graft matching specialist is available
- High-resolution digital images of matched allografts provided for your review
To submit a request for a matched graft, please visit our web-based Graft Matching Form below:

Sports Medicine Allografts
Tissue processing matters and data shows choosing the right allografts can improve patient outcomes
- Tendons processed aseptically maintain tissues natural biomechanical and biochemical composition
- Wide variety of sizes tailored towards surgeons needs and reduce OR time
- Supported by a compendium of clinical studies*
Featured Clinical Resources
Our clinical research drives our continuous improvement. Several peer-reviewed studies demonstrate that our commitment to aseptic processing – avoiding the use of terminal irradiation – results in reduced failure rates and greater cellular activity and incorporation compared to tissues treated with harsh processing methods.
Revision rates after anterior cruciate ligament reconstruction using bone-patellar tendon-bone allograft or autograft in a population 25 years old and younger.
Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft.
Clinical Dossier MTF Allograft Tendons for ACLR
Ordering Information
Contact MTF Customer Service at (800) 433-6576
For international enquiries, please contact +1 (732) 661-0202
MTF Biologics Completes First Implantation of FlexHD® Pliable in Milestone Breast Reconstruction Clinical Trial
March 26, 2025 —
Read more
